Roads Disrupt Frugivory and Seed Removal in Tropical Animal-Dispersed Plants in French Guiana
- 1UMR MECADEV 7179 CNRS-MNHN, LABEX DRIIHM, Département Adaptations du Vivant, Muséum National d’Histoire Naturelle, Brunoy, France
- 2Laboratoire Evolution et Diversité Biologique (UMR 5174), Université de Toulouse, Toulouse, France
- 3International Bird Conservation Partnership, Monterey, CA, United States
- 4Departamento de Ecologia, Instituto de Biociências, Universidade de São Paulo, Sâo Paulo, Brazil
- 5The Integrative Ecology Group, Estación Biológica de Doñana (CSIC), Seville, Spain
Ecological interactions are being affected at unprecedented rates by human activities in tropical forests. Yet, the continuity of ecological functions provided by animals, such as seed dispersal, is crucial for forest regeneration and species resilience to anthropogenic pressures. The construction of new roads in tropical forests is one of the main boosters of habitat destruction as it facilitates human access to previously isolated areas and increases defaunation and loss of ecological functions. It, therefore, becomes increasingly urgent to rapidly assess how recently opened roads and associated anthropogenic activities affect ecological processes in natural habitats, so that appropriate management measures to conserve diversity can be taken. In this study, we aimed to evaluate the effects of anthropogenic pressures on the health status of a mature rainforest crossed by a newly opened road in French Guiana. For this, we combined different methods to conduct a rapid assessment of the forest’s health status. Firstly, we evaluated the activity of frugivores using camera traps deployed in four forest patches located near (<1 km) ecological corridors preserved as canopy bridges over the road during the fruiting periods of four animal-dispersed tree species. Secondly, we analyzed the fate of seeds enclosed in animal-dispersed tropical fruits by calculating the proportions of fruits consumed and seeds removed (either dispersed or predated) by frugivores. Results show that the proportion of fruits opened and consumed was lower in the forest areas located near the road than in the control forest, and this difference was more significant for plant species strictly dependent on large-bodied primates for seed dispersal than for species relying on both primates and birds. Camera traps showed the presence of small primates and kinkajous feeding on Virola fruits in the forest impacted by the road, where large primates were absent. It is thus likely that smaller frugivores exert a compensatory effect that maintains ecological functions near the road. Despite efforts made to preserve forest continuity through ecological corridors, anthropogenic pressures associated with road proximity are affecting wildlife and disrupting associated ecological functions crucial for plant regeneration, contributing to further forest degradation.
Introduction
Roads facilitate economic development by increasing exchange and access to agricultural land and resources, but they have detrimental effects on natural ecosystems and human populations, especially indigenous people inhabiting remote forested areas (Campbell et al., 2017; Laurance and Arrea, 2017). As a result, most natural ecosystems and protected areas, located far from densely populated urban areas, are increasingly threatened by the rapid expansion of road networks worldwide, which favor rampant anthropogenic activities such as hunting, logging, mining, agriculture and encroachment of small-scale human activities (Laurance et al., 2012). Modern anthropo-ecosystems are becoming a matrix progressively surrounding fragmented protected areas and indigenous people’s land, increasing anthropogenic pressures both outside and inside (Laurance et al., 2001, 2012). For instance, the edges of the 100-km2 squared forest fragment of the Adolpho Ducke Forest Reserve near the city of Manaus (Brazil) were already visible early in the 1990s on Landsat images (Rodrigues et al., 2003). The dramatic report of collapsing protected areas calls for a new paradigm and strategy for road planning to preserve remaining natural tropical habitats. Such areas host most of the wildlife diversity upon which the recruitment of plants and the resilience of forest cover and carbon stocks depends (Laurance et al., 2014; Alamgir et al., 2017, 2020; Brodie and Fragoso, 2020; O’Bryan et al., 2020).
The effects of roads on forest fragmentation and species loss in natural ecosystems are well documented at different scales, particularly in temperate biomes. The negative impacts of road networks on wildlife communities have been reported, on average, five times as much as positive effects (Forman and Alexander, 1998; Trombulak and Frissell, 2000; Coffin, 2007; Fahrig and Rytwinski, 2009; Laurance et al., 2009). Road expansion increases the mortality rate of animals by introducing vehicles and collisions, reducing connectivity among populations, and allowing hunters and poachers better access to the forest. These road-mediated anthropogenic activities contribute to rapidly increasing defaunation of forest habitats, from hundreds of meters up to several kilometers from roadsides (de Thoisy et al., 2005; Laurance et al., 2009, 2012; Clements et al., 2014; Benítez-López et al., 2019; Gallego-Zamorano et al., 2020). The consequences of roads for ecosystem services also depends on species’ characteristics (reproduction rate, home range, migration, behavior), with larger animals overall being more impacted than smaller ones (Trombulak and Frissell, 2000; Peres, 2001; Nuñez-Iturri and Howe, 2007; Laurance et al., 2009; Boissier et al., 2020).
There is now a growing consensus to minimize the number of planned roads (Clements et al., 2018; Vilela et al., 2020) and implement so-called “green” or “ecological” corridors. Examples of these, in tropical habitats, are wildlife overpasses or underpasses and canopy walkways across roads. Corridors support the resilience and movement of animals alongside roads and across fragmented landscapes (Ng et al., 2004; Gregory et al., 2017; Shi et al., 2018), although their efficiency to reduce forest defaunation still needs to be evaluated. To our knowledge, few authors have addressed the consequences of changes in wildlife diversity for the ecology of native plants located in the vicinity of roads (Forman and Alexander, 1998; Wright and Duber, 2001; Coffin, 2007; Laurance et al., 2009).
Located in northern South America, the Guianas ecoregion remains well preserved and non-fragmented in comparison to the rest of the Amazon basin (Huber et al., 2002), with remote and isolated protected areas surrounded by vast tracts of mature forests (>50 km in width, sensu Laurance et al., 2012). However, anthropogenic pressures in the Guianas are steadily increasing due to the renewed demand from the economic sectors for more roads and infrastructure that bisect and fragment large patches of mature forest, facilitating the exploitation and exportation of natural resources (Hammond, 2005). To establish efficient conservation programs for the resilience of biodiversity to environmental threats such as roads and the anthropogenic activities they promote, it is thus important to assess the current health status of tree species in forested habitats that are crossed by new roads (Boissier et al., 2020).
As in other ecosystems, many tropical forest trees rely on animals for seed dispersal (Markl et al., 2012; Carreira et al., 2020; Emer et al., 2020). The health of tropical forests, therefore, depends on the resilience of frugivore-plant interaction networks to anthropogenic pressures such as fragmentation, selective logging, and hunting (Kitamura and Poonswad, 2013; Corlett, 2017; Dugger et al., 2019; Naniwadekar et al., 2019). Traits such as animal body mass, gape width, animal behavior, fruit morphology and seed dimensions determine whether seeds are swallowed, spat, dropped or carried, and thus whether they will be dispersed further away from parent plants by frugivores (Forget et al., 2007; Naniwadekar et al., 2019; Sivault et al., 2020). Therefore, for evaluating seed dispersal effectiveness is also necessary to estimate the role of smaller-bodied frugivores that may contribute as efficient alternate seed dispersers once the major larger-bodied frugivores are lacking or extinct (Carreira et al., 2020). In French Guiana, for instance, despite an overall reduction in seed removal rates at the plant community level due to modifications of the frugivore community, defaunation differentially impacted seed removal rates between plant families (Boissier et al., 2020).
Using a rapid assessment method to evaluate seed removal, Boissier et al. (2020) observed that the downsizing effect of hunting was greater for plant species in the primate-dispersed family Sapotaceae than for those in the bird-and-primate-dispersed Burseraceae and Myristicaceae. The compensation of the loss of primates was possibly explained by the resilience of birds, especially Ramphastidae (toucans), to anthropogenic pressures (see Holbrook and Loiselle, 2009 for similar results). Using the same method, Hambuckers et al. (2020) observed that the amount of forest cover in an overall fragmented landscape affected seed removal rates, which also differed between two frugivore-dispersed species. They concluded that the health of the forest fragments was similar to that of a disturbed forest, validating the use of seed removal rate as an indirect indicator of forest health and as a proxy to evaluate the activity of frugivores of all body sizes (Nuñez-Iturri and Howe, 2007; Gutierrez-Granados and Dirzo, 2010; Levi and Peres, 2013).
Our present study aims to monitor and analyze the ecological consequences of road-mediated anthropogenic activities along a recently opened road in French Guiana and the compensatory effect of ecological corridors. More specifically, we aimed to evaluate how “green bridges,” connecting the recently bisected forest, can effectively contribute to the resilience of ecological services such as animal-mediated seed dispersal. Firstly, we hypothesized that reproductive trees located closer to the road and urbanized areas will face greater anthropogenic pressures (traffic, hunting) than trees located further away or in intact forests. Secondly, we hypothesized that plants relying on a specialized seed dispersal system with fewer large frugivores will be more greatly impacted by road effects than those with a generalized dispersal system comprising a community of small-to-large frugivores (Howe, 1993). To test these hypotheses, we used rapid assessments (Boissier et al., 2014) for evaluating the levels of fruit consumption and seed removal of four animal-dispersed plants, namely Virola micheli, V. kwatae (Myristicaceae) and Manilkara huberi and M. bidentata (Sapotaceae) at the tree population level. In addition, we placed camera traps on the ground and in the canopy of focal fruiting trees to evaluate the diversity of wildlife in the forest areas surrounding the ecological corridors. Results of the forest impacted by the road are discussed and evaluated in comparison to a protected (Nouragues) and another disturbed forest (Montagne de Kaw), where the same tree species and wildlife co-occur.
Materials and Methods
Study Sites
French Guiana is a French overseas department located in South America, bordered by Brazil in the East and by Suriname in the West. The climate is humid equatorial with a long rainy season between November and August, eventually interrupted by a small dry season in February or March, followed by a dry season between September and October. The development of road networks in the 1990s included a project of socio-economic development in Eastern French Guiana to improve the mobility of goods and people between the department and the border with Brazil, as well as offer better access to timber and agricultural lands (Boudoux d’Hautefeuille, 2010).
The study area is a mature rainforest growing along that road, named “Nationale 2” (hereafter RN2; Bens, 2011; Pérez and Archambeau, 2012; Nicolle and Boudoux d’Hautefeuille, 2014) extending from Cayenne (capital city of the department) on the littoral to Saint-Georges in the interior along the Oyapock river, which marks the border between French Guiana and Brazil (Figures 1A,B and Table 1). In 2017, a bridge connecting French Guiana to Brazil by land was built (Pérez and Archambeau, 2012) and officially opened to traffic, with likely further social, economic and environmental effects on both the inhabitants of Saint-Georges and the forest located along the road (Grenand, 2011). To limit the fragmentation effects and to maintain forest connectivity above the new section of RN2, a total of 13 ecological corridors were created during the opening of the former continuous forest block with tree cover being preserved near roadsides aiming at connecting the two edges of the bisected canopy cover. However, due to degradation of the vegetation cover at both edges as well as inside the corridors, along with tree falls, natural or man-made (to avoid accidents), connectivity is currently far from being complete.
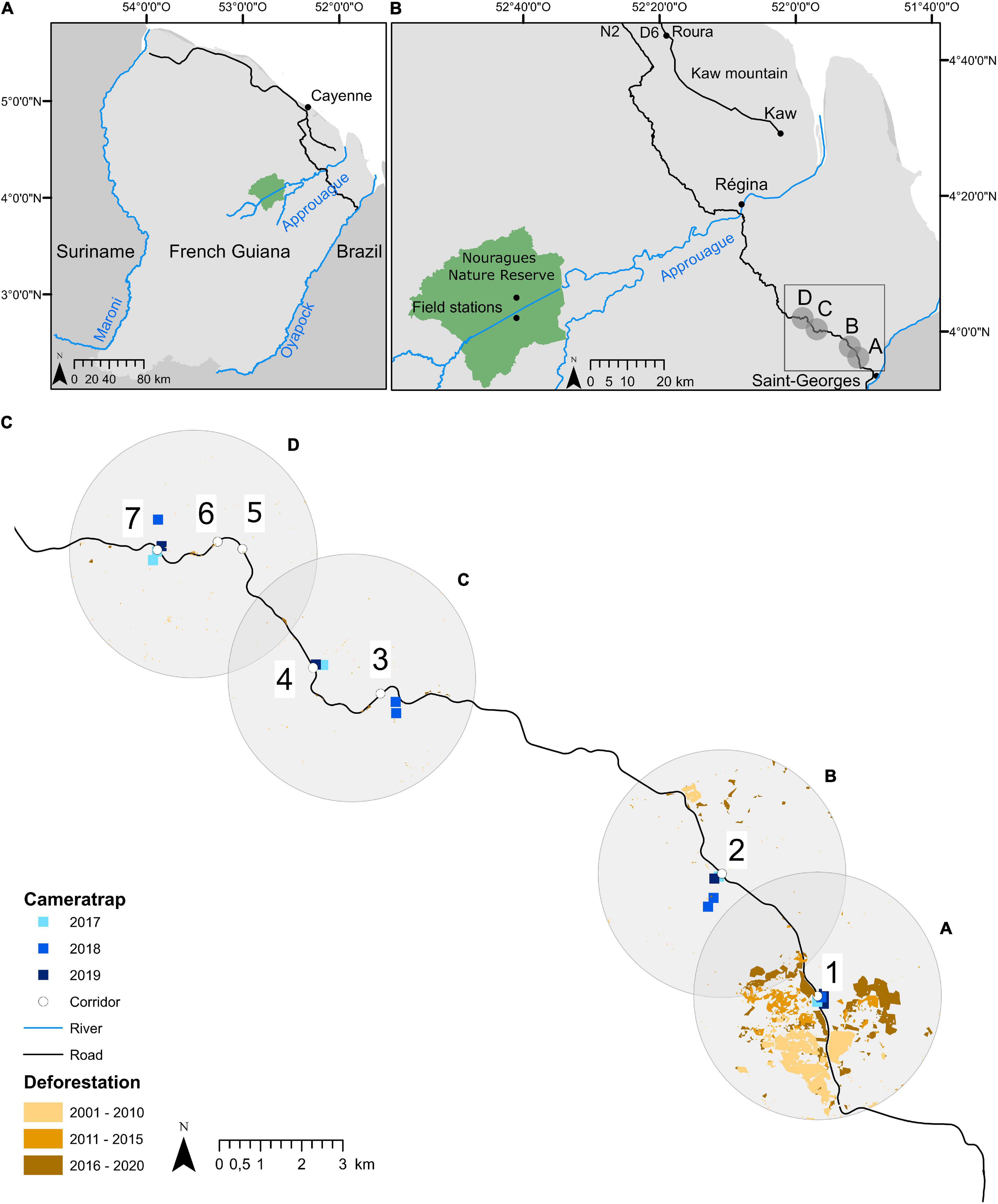
Figure 1. (A) Map of French Guiana, in northern South America; (B) regional map showing the 3 study areas: Nouragues Nature Reserve, Montagne de Kaw, and Road “Nationale 2” (RN2) between Cayenne and Saint-Georges; (C) details of the location of the camera traps installed on the ground beneath fruiting Virola spp. and Manilkara spp. trees at the four forest patches along the new RN2 section between Régina and Saint-Georges. For the purpose of this study, study trees were located in forest patches surrounding the ecological corridors within 1 km from the road and up to ca. 33 km from the Oyapock River at Saint-Georges (the exact location of study trees is not shown for security reasons).
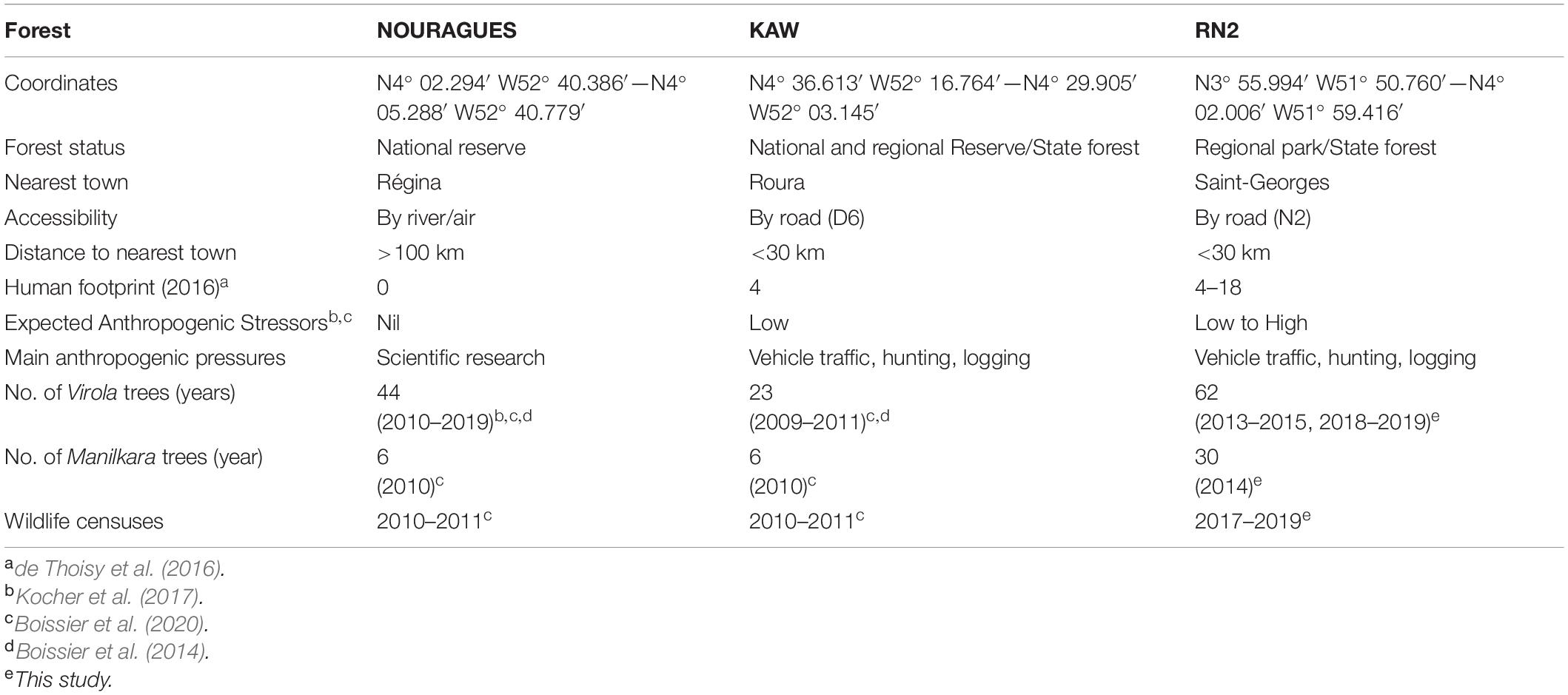
Table 1. Study sites in French Guiana, with hypothesized increasing intensities of anthropogenic disturbance (NOURAGUES, Nouragues Field Station; KAW, Montagne de Kaw; RN2, Road “Nationale 2”).
Observations and fruit surveys were carried out in the forest surrounding seven out of the 13 corridors at various distances along RN2 (Figure 1C, Supplementary Figure 1, and Supplementary Table 1). Study sites were all located on hilltops (where the road opening was created to avoid slopes and flooded terrain), generally providing easy access to the mature forest nearby. Forest patches near corridors were chosen after an intensive search for the fruiting study tree species (Figure 1). Four areas were defined referring to forest surrounding corridors 1 (hereafter “A”), 2 (“B”), 3 and 4 (“C”) and 5, 6, and 7 (“D”) (Figures 1B,C, Supplementary Figure 1, and Supplementary Table 1). Elevation increased along RN2 with distance from Saint-Georges (Supplementary Figure 1). Forest patches A and B were located near logged forests, while C and D were part of the Parc Naturel Régional de Guyane, a partially protected area with high forest cover and low deforestation rates (Figure 1C and Supplementary Figure 1).
The forest at study sites was characterized by hilly topography. Hilltop forests present a species richness and a species composition different from downslope forests, as frequently observed in the Guianas (Guitet et al., 2015). Forest types encountered at the study area are described as a mosaic of ecotone and mixed forest types mainly characterized by the abundance of Fabaceae, Sapotaceae, Chrysobalanaceae, Burseraceae and Lecythidaceae (Forget, pers. obs.). Canopy height is between 30 and 40 m, with some emergent trees. The type of geomorphological landscape types is referenced as “High Plateaux or Tableland” and “Mountains” (so-called “Montagne”) with a mean altitude between 50 and 200 m (e.g., categories G and H, respectively, in Guitet et al., 2015; Guitet et al., 2018). Despite the increasing impact of hunting and other sources of human disturbance, wildlife encountered along RN2 ten years ago led the forest to be regarded as relatively undisturbed, compared to that of the Nouragues Nature Reserve (hereafter Nouragues, Figure 1B; de Thoisy et al., 2010, 2016). However, since the construction of the new section of the RN2 between Régina and Saint-Georges, ca. 20 years ago, the forest became more accessible, and recent studies suggest a recent decrease in species richness and abundance of vertebrate wildlife at a more local scale (Kocher et al., 2017, 2022). This would be comparable to that of Montagne de Kaw (hereafter Kaw, Figure 1B) with a impoverished population of large-bodied fruit-eating primates (Boissier et al., 2020; P.-M. Forget, pers. obs. in 2013–2022).
The study area was characterized with a theoretical anthropogenic gradient extending from the Oyapock river and Saint-Georges to the interior (Figures 1B,C and Supplementary Figure 1), with A and B being the closest (< 20 km) and likely the most disturbed, and C and D the farthest (> 20 km) and least disturbed in accordance with the Human Footprint Index (HFI ranging 4–18; de Thoisy et al., 2010, 2016). Moreover, we also expect an anthropogenic gradient extending from the RN2 to the interior; along this gradient, forest areas located closer to the road should be more disturbed than forest areas located further away from the road. Anthropogenic pressures thus increase with forest accessibility, here defined by two parameters: (1) the distance of trees to the RN2; and (2) the distance of the trees to Saint-Georges (Supplementary Table 1).
Data collected at RN2 were analyzed in comparison with other datasets collected in forests at Nouragues (control, HFI = 0) and Kaw (disturbed, HFI = 4) (sites 3 and 4, respectively, in de Thoisy et al., 2016), where the latter comprises the Tresor Regional Nature Reserve (Boissier et al., 2014, 2020; Table 1).
Study Trees
We studied two wild nutmeg trees Virola kwatae Sabatier, 1997, and V. michelii Heckel, 1898 (Myristicaceae), and two canopy bulletwood trees (or balata) Manilkara bidentata (A.DC.) A. Chev., 1932 and Manilkara huberi (Ducke) A. Chev., 1932 (Supplementary Figure 2 and Supplementary Table 2). Virola trees are annual dioecious species that fruit between November and March in French Guiana (Ratiarison and Forget, 2013; Mendoza et al., 2018). Virola species produce capsule-like fruit possessing two valves protecting a seed covered by a lipid-rich aril (Figure 2). When fruits reach maturity, they naturally dehisce into two valves exposing the red-colored aril. The two valves are firmly attached to each other and do not separate when falling without being handled or processed by a diverse coterie of both arboreal, flying and ground-dwelling vertebrate frugivores (Supplementary Table 3). Virola kwatae trees grow almost exclusively uphill, on ridges and mountains, and produce larger seeds (seed size average: 2.8 × 1.8 cm) than V. michelii (seed size average: 2.0 × 1.4 cm), thus attracting larger frugivores. Due to their large size, V. kwatae seeds are mainly dispersed by the black spider monkey (Ateles paniscus), kinkajous (Potos flavus) and toucans (Rhamphastos spp.). Virola kwatae was indeed named after the black spider monkey (called “kwata” in the bushi-nenge vernacular name) because it is the main fruit consumer and seed disperser (Forget and Sabatier, 1997; Sabatier, 1997). Other consumers such as red howler monkey (Alouatta macoonelli) and green oropendola (Psarocolius viridis) only play a minor role in the dispersal of V. kwatae’s seeds. The other tree species, V. michelii, is also dispersed by spider monkeys, howler monkeys, kinkajous and toucans. In addition, the smaller seed size of V. michelii allows dispersion by brown capuchin (Sapajus apella) and birds such as marail guan (Penelope marail) and the Amazonian motmot (Momotus momota). The arboreal mammalian frugivores (e.g., Primates) have prior access to seeds and can consume them before they naturally dehisce. Indeed, these animals can handle and open fruit before dehiscence, reach the arilled seed, swallow it, and drop single-valved fruit to the ground. On the contrary, birds have to wait for fruit dehiscence to reach the edible aril, and valves remain sealed when dropping after fruit abscission (Boissier et al., 2014).

Figure 2. Fruits and seeds of (A) Virola kwatae (left) and V. michelii (right), and (B) Manilkara bidentata (left) and M. huberi (right) (all photographs © Pierre-Michel Forget).
Manilkara tree species are common trees, 40–50 m tall, with mast fruiting behavior (Norden et al., 2007; Mendoza et al., 2018; Supplementary Table 2). Fruits are ripe between March and June, i.e., during the fruit peak at the community level, when ground-dwelling consumers are satiated with fruits and seeds on the ground (Mendoza et al., 2015; Supplementary Table 3). Such satiation increases the probability of seed survival (Mendoza et al., 2015), and thus favors seedling recruitment (Chauvet et al., 2004). Manilkara fruits are drupes containing one to five medium-sized seeds (seed size average: 2.4 × 1.3 cm) embedded in sugar-rich pulp (Figure 2). Fruit and seed traits differ between M. bidentata and M. huberi, with fruits of the latter being more globose, containing a slightly greater mean number of seeds (1.1 vs. 1.6 on average, respectively) and thus being heavier than the former. During the study, the average weight of fruit and seeds was established to be 14.7 and 9.5 g, respectively, for M. bidentata, and 1.7 and 1.2 g, respectively, for M. huberi (Ratiarison and Forget, 2011). Fruits of both Manilkara are consumed by a diversified community of frugivores and granivores, with the main diurnal arboreal consumers and seed dispersers being primates in the undisturbed forest of the Nouragues Nature Reserve (Zhang, 1995; Ratiarison and Forget, 2011). Ground-dwelling consumers of Manilkara fallen fruits are partially known (Chauvet et al., 2004), and include small spiny rats (Proechimys cuvieri and P. guyannensis), medium-to-large rodents (Myoprocta acouchy and Dasyprocta leporina) and peccaries (Tayassu pecari and Pecari tajacu).
Frugivore Data Collection by Camera Trapping
Camera traps from the brand Reconyx ®, model HC600 equipped with a red infrared moving sensor, were placed at Virola and Manilkara trees in the forest surrounding corridors to characterize the community of frugivores (Figure 1C and Table 2). Ground cameras were set a few meters away with the lens pointing to the base of fruiting individuals of Virola (2017–2019) and Manilkara (2017, during a masting event), and attached at nearby tree bases, ca. 50 cm above the ground. For Virola, camera traps were installed during 2017, 2018 and 2019 fruiting seasons, after fruit sampling. In 2019, the tree crowns of Virola trees were accessed using a single rope climbing method (Picart et al., 2014), and cameras were placed with the lens pointing to large branches possessing fruit at the extremity (Moore et al., 2021). The cameras were then left operational for 6 weeks from early February until mid-March. For Manilkara, camera traps were installed in 2017, on trees that were sampled for fruit during the masting event of 2014. Cameras were set up to take five pictures per trigger, with the shortest trigger delay setting and the shortest time setting between successive pictures. The sensor was set at its highest sensitivity. To minimize untimely triggering, which occasionally occurs on the ground and very often in the canopy due to leaf movements, incipient plants, leaves and small branches were cut when they were too close to the camera (around less than 3 m). The shortest distance between two camera traps within forest patches was 225 m. This was in the most disturbed forest where it was difficult to circulate away from the tracks and where study fruiting trees were scarce. The number of cameras installed per fruiting season, the settling and removal days, as well as the length of the recorded periods, are detailed in Table 2. Supplementary Table 3 lists vertebrates known to consume fruits and seeds of the study tree species.
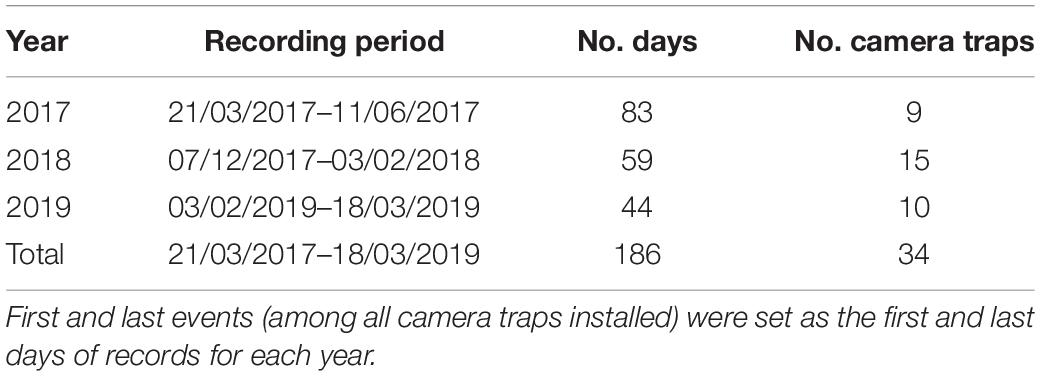
Table 2. Camera trap selection for characterizing the ground-dwelling fauna in the entire studied area including forest patches A, B, C and D along the National Road 2 (NR2) between Régina and Saint-Georges, French Guiana.
Fruit Consumption and Seed Removal
Trees were searched for at locations where they were most likely to be present, using hunting trails and walking along ridges (Supplementary Table 4). Fruit surveys were conducted toward the end of the fruiting season of Virola (late January/early February 2009–2019) and during a masting year of Manilkara (late May and early June 2014) trees. To count fallen fruits and seeds, 1-m2 quadrats were randomly placed on the ground 2–3 m from each trunk base (one quadrat per tree from 2010 to 2015 and three per tree from 2017 to 2019, following Hambuckers et al., 2017). This distance avoided sampling areas under branches not bearing fruits, where fruits were less numerous on the ground than expected at random.
Fruits and seeds of Manilkara were sampled underneath tree crowns following the method described in Boissier et al. (2020). Within each quadrat, intact fruits were collected and counted. To determine the number of eaten fruits, husk parts were collected, air-dried for 24 h with an indoor electricity-powered fan, then weighed. The weight of the husk parts was divided by the average weight of Manilkara fruits (M. huberi: 14.73 g; M. bidentata: 9.55 g). The number of single, unpredated seeds was also counted. Estimates of the total numbers of seeds were established for M. bidentata and M. huberi using the average of 1.1 and 1.6 seeds per fruit, respectively (Ratiarison and Forget, 2011). Since seed removal can be affected by the fruit crop, percentages were determined from the total number of fruits within each quadrat (Boissier et al., 2020).
For both species and within each quadrat, all conspecific fruits and seeds were counted and categorized as (1) intact fruit, (2) eaten fruit, (3) dehisced fruit with both valves still joined together (only for Virola), (4) single valve (only for Virola) or (5) seed, according to Boissier et al. (2014) and Boissier et al. (2020). Three indices were then calculated from these estimates. (1) The fruit consumption rate (proportion of eaten fruits) in Manilkara is calculated as the number of eaten fruits divided by the total number of fruits. (2) The proportion of fruits found as single valves in Virola is half the number of single valves (two valves per fruit) divided by the total number of fruits. Both these ratios are proxies to evaluate the activity of arboreal and ground-dwelling consumers and their consumption of fruits. Finally, (3) the seed removal rate is the proportion of seeds that were removed by animals, either dispersed or predated, and are thus missing from the quadrat. It is based on the proportion of remaining seeds, which is calculated as the total number of seeds remaining in the quadrat (either found on the ground or still contained in intact fruits) divided by the total number of seeds expected to be found in the quadrat if none had been taken away by animals (based on the total number of fruits found in the quadrat and the number of seeds per fruit, one for Virola and a mean observed number for Manilkara). In Virola, consumption of entire ligneous fruits is unlikely, only the fleshy aril and seeds are consumed, swallowed or destroyed by consumers. In Manilkara, the pericarp is not swallowed in the canopy, then it is dropped beneath the fruiting tree after the pulp surrounding the seeds has been consumed by arboreal frugivores. Uneaten fruits fall entire on the ground where they accumulate when not consumed by ground-dwelling vertebrates. Hence, the seed removal rate is calculated as one minus the proportion of remaining seeds. The quadrat method had proven to be an efficient field “tool” in order to calculate an index of fruit consumption and seed removal below fruiting plants for the two study genera, and more details and formulae can be found in Ratiarison and Forget (2013), Boissier et al. (2014), and Boissier et al. (2020).
Fruit sampling of all species was conducted in 2010, 2011, and 2019 at the two field stations Inselberg and Pararé at Nouragues (Figure 1B), and in 2013, 2014, 2015, 2017, and 2018 at RN2. Fruit data from 2010 to 2015 were collected exclusively using one quadrat rather than the three replicates used from 2017 onward. We assessed the effect of the number of quadrats by comparing fruit consumption and seed removal calculated from one quadrat vs. 3 quadrats (for each tree sampled with 3 quadrats) using Student and Mann–Whitney U-tests for paired samples. There was no effect of the number of quadrats (Mann–Whitney U rank test, U = 341, p = 0.28, n = 35; Student test, t(34) = 0.22, p = 0.83, n = 35) on fruit consumption and seed removal.
Data Analyses
The package camtrapR was used to organize and extract data from the raw images (Sollmann et al., 2016). DigiKam software was chosen to add metadata to the images. Data were extracted into a record table for each year. We took a conservative approach and we considered records as different events when the time difference between records of the same species at the same station was larger than 60 min.
To characterize the community of frugivores feeding on Virola and/or Manilkara fruits and/or seeds, we removed all events of species not consuming or predating fruits or seeds, and we compiled all of the events recorded during 2017, 2018, and 2019 (Table 2). The package iNEXT (Hsieh et al., 2016) was used to compute species accumulation and coverage-based (i.e., sample completeness) sampling curves as well as an estimation of species richness based on the method proposed by Chao et al. (2014).
We used percentages of fruit consumption, fruits found as single valves and seed removal rate as proxies for the activity of both frugivores and seed predators in the canopy and underneath the tree crown. After testing for a species effect on these proxies using Kruskal-Wallis rank-sum tests, proxies were compared between forest patches and between Nouragues, Kaw and RN2 forests with Kruskal-Wallis rank-sum tests and Pairwise-Wilcoxon statistical tests.
Generalized linear mixed models computed with the lmer function from the package lme4 (Bates et al., 2015) were used to test for an effect of the distance to the road and the distance to Saint-Georges on the proportion of fruits found as single valves (for Virola), on the fruit consumption rate (for Manilkara), and on seed removal rate (for both genus). The year of sampling, and the tree species were used as random effects to account for temporal and species-specific variability for Virola while species only was used as a random factor for Manilkara since the sampling was only conducted in 2014. As the response variable was proportion data, we used a binomial error distribution with a logit link, with the number of fruits implemented as the weight argument. Data analyses were performed with R 3.2.3 (R Core Team, 2021).
Results
Frugivore Community
After camtrapR analysis and selection, 770 independent events of frugivore visits were recorded corresponding to 16 species (Table 3 and Supplementary Tables 5, 6). The species accumulation curve of ground and arboreal frugivorous species did not reach a plateau at the end of the survey period (Figure 3A). Sampling effort (186 days of records) allowed a sample coverage of 95.2% (Figure 3B) and the Chao estimation of species richness was 18.98 ± 4.53 se.
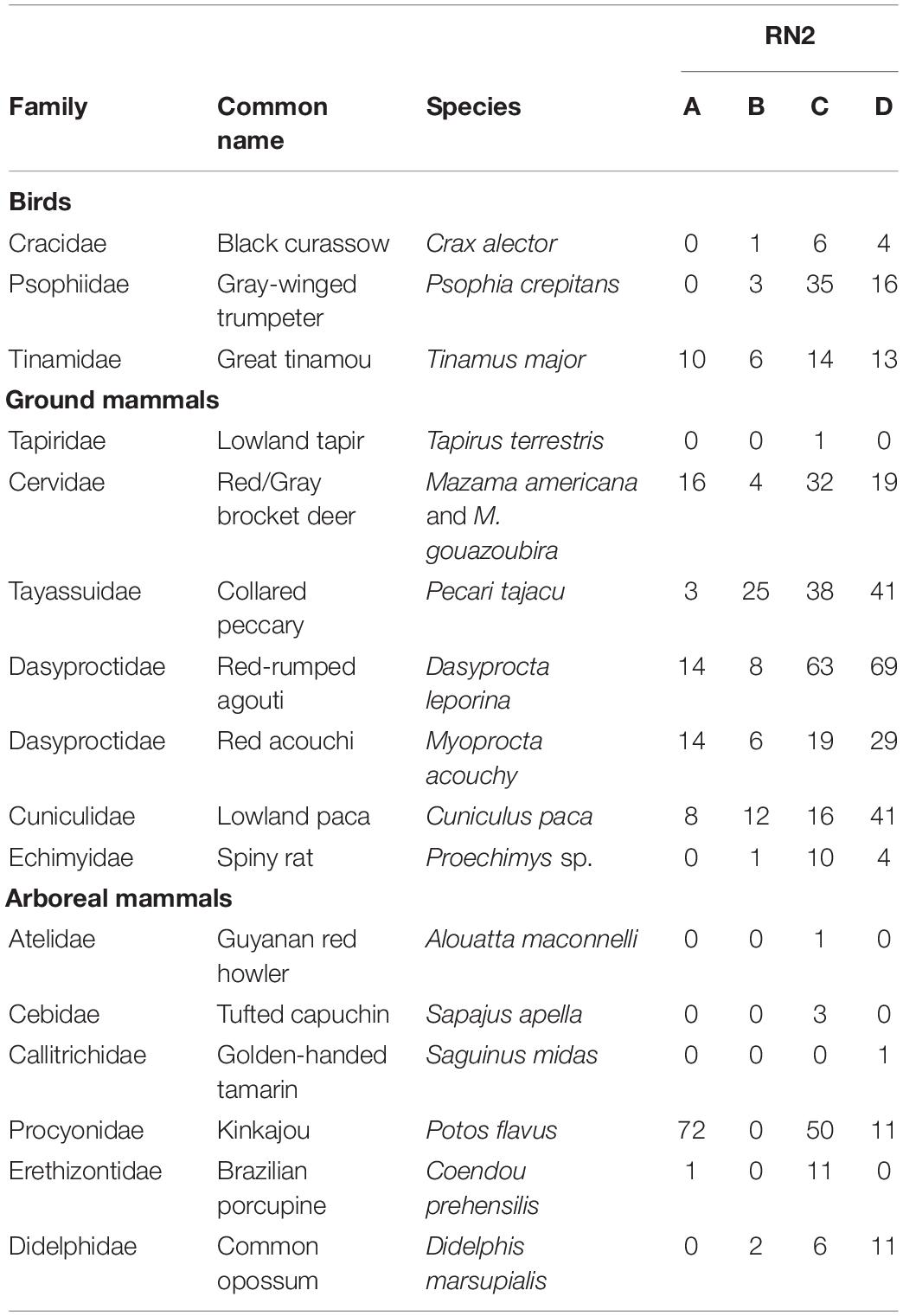
Table 3. Numbers of detection events of frugivorous species recorded near and in Virola and Manilkara trees censused with Reconyx ® camera traps at the four forest patches along Road “Nationale 2” (RN2), between Régina and Saint-Georges, French Guiana.
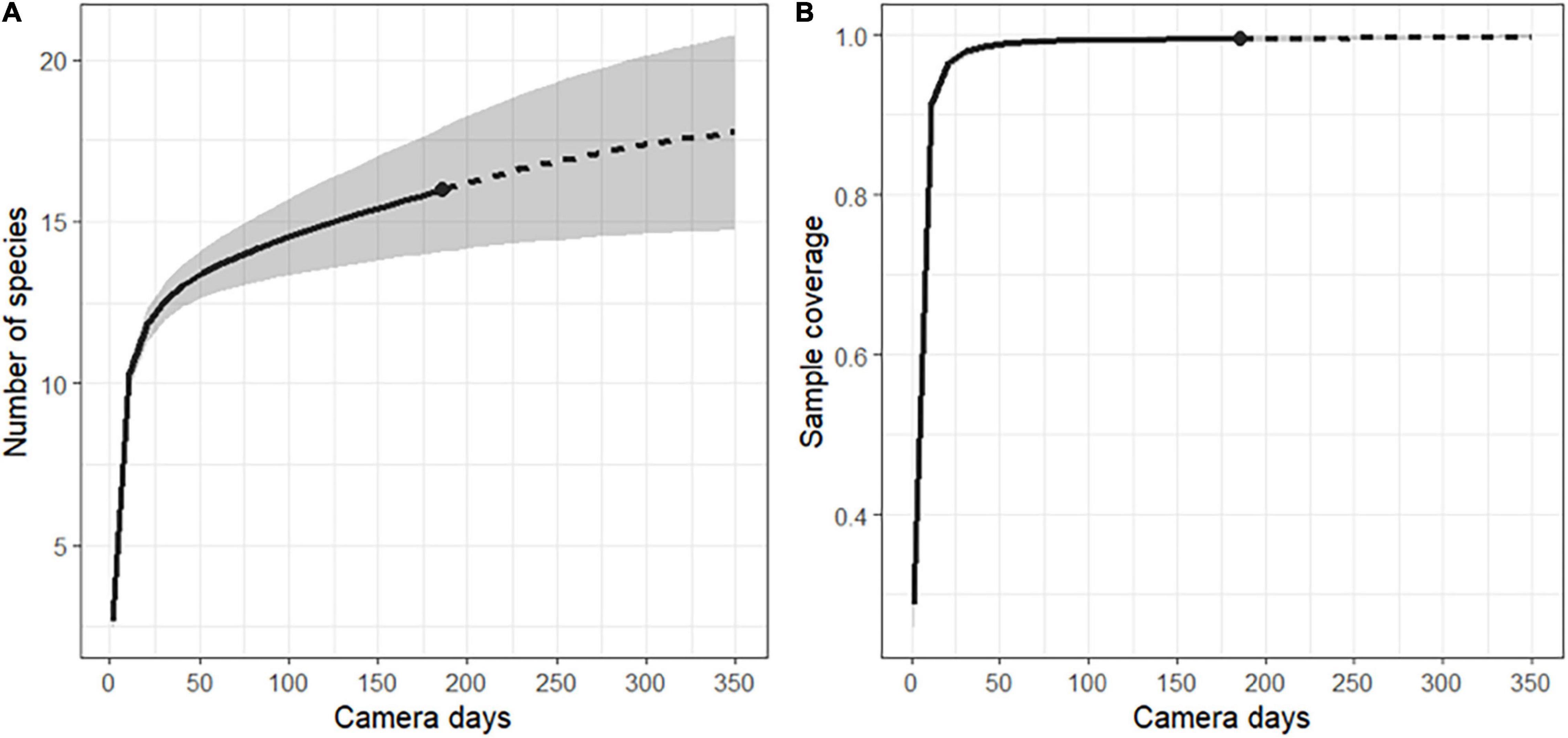
Figure 3. (A) Randomized species accumulation curve and (B) coverage-based sampling curve for the ground and arboreal frugivore community of the entire study area (including forest patches A, B, C, and D) recorded in the vicinity of Manilkara and Virola trees. The shaded area represents the 95% confidence interval. The dot at the limit between the interpolated solid line and the extrapolated dashed line indicates the actual sampling effort of the study (186 days of record).
All major known ground-dwelling frugivores of the study species were observed (Table 3). Beneath Virola spp., we recorded red-rumped agouti (Dasyprocta leporina), collared peccari (Pecari tajacu) and brocket deer (Mazama gouazoubira and M. americana), which were grouped in this study because they are almost indistinguishable in photographs (Figure 4). At Manilkara trees, frequent consumers were the gray-winged trumpeter (Psophia crepitans), great tinamou (Tinamus major), black curassow (Crax alector), red acouchy (Myoprocta acouchy), lowland paca (Cuniculus paca) and lowland tapir (Tapirus terrestris, Figure 5). On Virola spp. tree crowns, arboreal frugivores such as the kinkajou (Potos flavus), tufted capuchin (Sapajus apella), red howler monkey (Alouatta macconnelli) and Golden-handed tamarin (Saguinus midas) were observed. In addition, two species not known to feed on Virola fruits and seeds were recorded: the common opossum (Didelphis marsupialis) and the Brazilian porcupine (Coendou prehensilis). The arboreal kinkajou was the major visitor of Virola and corresponded to 55% of visits in the 2019 survey (Supplementary Figure 3).

Figure 4. Some camera trap images of the ground-dwelling frugivores observed beneath fruiting Virola kwatae and V. michelii trees in forest patches along Road “Nationale 2” (RN2) between Régina and Saint-Georges, French Guiana, in February 2015 and December 2017: (A) Red-rumped agouti (Dasyprocta leporina); (B) collared peccary (Pecari tajacu); (C) gray brocket deer (Mazama gouazoubira); (D) red brocket deer (Mazama americana).

Figure 5. Some camera trap images of the ground-dwelling frugivores observed beneath fruiting Manilkara huberi and M. bidentata trees in forest patches along Road “Nationale 2” (RN2) between Régina and Saint-Georges, French Guiana, in March–May 2017: (A) Gray-winged trumpeter (Psophia crepitans); (B) great tinamou (Tinamus major); (C) black curassow (Crax alector); (D) red acouchy (Myoprocta acouchy); (E) lowland paca (Cuniculus paca); (F) lowland tapir (Tapirus terrestris).
Single-Valved Fruits, Fruit Consumption, and Seed Removal
A total of 62 Virola and 30 Manilkara trees were sampled at RN2 between 2013 and 2019, and in 2014, respectively (Supplementary Tables 4, 7, 8).
There was no significant effect between Virola species for either percentage of fruits found as single valves (V. kwatae: average 42.35%; V. michelii: 32.60%) or seed removal (V. kwatae: average 76.11%; V. michelii: 68.88%) along RN2 (X2 = 3.81, df = 1, P = 0.14 and X2 = 2.15, df = 1, P > 0.05, respectively). The proportion of Virola fruits found as single valves and seed removal rate did not differ between forest patches (X2 = 0.73, df = 3, P = 0.09 and X2 = 0.65, df = 3, P = 0.33, respectively). Overall, the mean proportion of fruits found as single valves at RN2 (37.6%) was similar to Kaw (37.7%) and significantly lower (P < 0.001) than at Nouragues (66.1%). Similarly, the mean seed removal rate was significantly lower (P < 0.01) at RN2 (71.9%) than at Nouragues (83.8%), whilst intermediate at Kaw (81.46%; Figure 6).
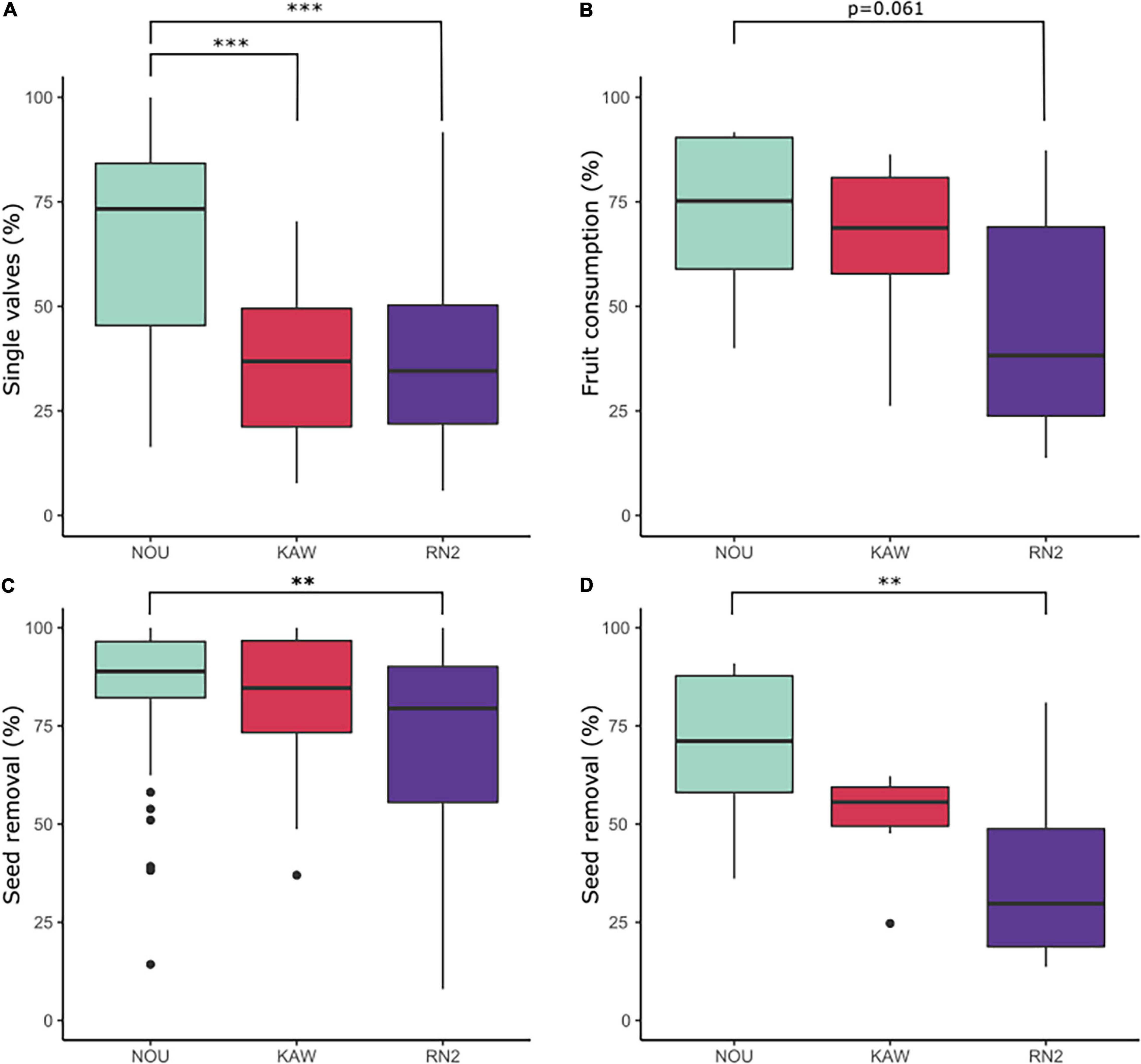
Figure 6. Box-plots representing indices of fruit fate and seed removal at Nouragues field station (NOU), Montagne de Kaw (KAW) and along Road “Nationale 2” (RN2): (A) percentage of Virola spp. fruits found as single valves; (B) percentage of Manilkara spp. fruits consumed (fruit consumption rate); (C) percentage of seed removal of Virola spp. and (D) Manilkara spp. Percentages were compared with Kruskal-Wallis rank-sum tests and a Pairwise-Wilcoxon statistical tests. ***p < 0.001; **p < 0.01.
There was no significant effect between Manilkara species for either fruit consumption (M. bidentata: average 43.8%; M. huberi: 46.1%) or seed removal (M. bidentata: 30.7%; M. huberi: 41.4%) along RN2 (X2 = 0.12, df = 1, P = 0.72 and X2 = 1.30, df = 1, P = 0.25, respectively). The proportion of Manilkara fruit consumption and seed removal rate did not differ between forest patches (X2 = 6.44, df = 4, P = 0.17 and X2 = 5.75, df = 4, P = 0.21, respectively). The mean proportion of Manilkara fruit consumed at RN2 (44.9%) was marginally significantly lower (P = 0.06) than at both Nouragues (71.8%) and Kaw (64.8%). The mean seed removal rate at RN2 (36.1%) was significantly lower than at Nouragues (69.4%), with Kaw (51.1%) being intermediate (P < 0.01; Figure 6).
Effects of Road and Distance to Urbanization
We analyzed the road effect (distance between trees and the road) and the distance to Saint-Georges on the proportion of fruit found as single valves (for Virola), on the fruit consumption rate (for Manilkara) and on seed removal rates (for both genera).
For Virola trees, the mean distance of trees to the road and to Saint-Georges was 403.9 m (range 10.32–894.88) and 25.08 km (range: 7.94–32.45), respectively. There was a significant and positive effect of the distance to the road (Z = 6.14, P < 0.001, slope = 1.03 ± 0.17; mean ± se) on the proportion of fruit found as single valves. We also reported a significant but very weak positive effect of the distance of trees to Saint-Georges (Z = 5.40, P < 0.001, slope = 0.04 ± 0.01) on the proportion of single valve fruits. The seed removal rate was significantly but weakly affected by the distance to the road (Z = 4.01, P < 0.001, slope = 0.67 ± 0.17), while no effect of the distance to Saint-Georges was reported.
For Manilkara trees, the mean distance of trees to the road and to Saint-Georges was 243.21 m (range 3.31–604.46) and 25.49 km (range: 7.94–32.45), respectively. There was a significant but weak positive effect of the distance to Saint-Georges (Z = −2.49; P < 0.05, slope = 0.02 ± 0.01) on the fruit consumption rate while no effect of the distance to road was reported. In addition, there was a significant but weak negative effect of the distance to Saint-Georges (Z = −2.13; P = 0.03, slope = −0.02 ± 0.01) on seed removal rate, while no effect of the distance to the road was reported.
Overall, there was no significant effect of distance to urban areas for either fruit consumption or removal in all study species.
Discussion
Ecological factors that can act on the visitation of fruiting trees by frugivores are forest type, tree accessibility, fruit occurrences and crop size (e.g., for Primates: Stevenson et al., 2005; Stevenson, 2016). In this study, we compared two seed dispersal systems in four tree species that co-occur in the same forest type (plateau and hilltop). They are all defined as specialized (sensu Howe, 1993) and produce large fruit and seeds that are dependent on large frugivores, especially primates and birds, for seedling recruitment away from parents. Abundant literature already demonstrated that the impact of human activities, especially hunting of the main fruit consumers of the study species, could alter their seed dispersal, but no study focused on roads and corridors (Pardo et al., 2019). We investigated here the alteration of these dispersal systems by the construction of a recent road fragmenting and facilitating access to the forest and therefore, promoting road-mediated anthropogenic activities such as hunting, logging and ranching.
A theoretical anthropic gradient was predicted along RN2 with various human activities and pressures across four forest patches, expected to decrease with increasing distance to Saint-Georges. After the bridge opened to traffic across the frontier French Guiana-Brazil in 2017, an increase of anthropogenic pressures along the RN2 was expected, notably with an increase in population and settlements along the road, as well as a development of human activities. We then expected biodiversity and the seed dispersal ecological process to be markedly impacted in the short term (de Thoisy et al., 2010).
Virola Seed Dispersal System
On the one hand, the absence of a significant difference in seed removal rate in Virola spp. across forest patches may indicate that seed dispersal is not yet markedly impacted by the predicted anthropic gradient along the RN2. Nevertheless, the lowest rate and the highest variance of seed removal compared to that of the Nouragues demonstrate that seed dispersal and predation occur more heterogeneously along the RN2 than at Nouragues. On the other hand, the high proportion of Virola fruits found as single valves at Nouragues, compared to both RN2 and Kaw, suggests that primate density and activity are significantly higher in the control forest, or lower in the environmentally stressed habitats such as a forest close to roads (Boissier et al., 2014, 2020). This higher proportion of Virola fruits found as single valves at Nouragues is best explained by the high abundance of black spider monkeys in this nature reserve, which is not a common species in RN2 (observed only once from 2013 to 2019; de Thoisy et al. (2005); P.-M. Forget, pers. obs.), and absent at Kaw (Boissier et al., 2020). This primate species is indeed very sensitive to hunting pressure and habitat quality (Nuñez-Iturri and Howe, 2007; Sales et al., 2020) and is one of the first species disappearing with anthropogenic pressures (de Thoisy et al., 2010). The presence of single-valved fruits at the RN2 thus results from the activity of other canopy-foraging animal species that can manipulate fruits to reach the aril, such as the red howler monkey and brown capuchin, despite them being known to have a minor role in Virola spp. seed dispersal at the Nouragues control forest (Ratiarison and Forget, 2013).
In addition, camera traps placed in the canopy revealed that kinkajou is the most frequent visitor of Virola trees (Supplementary Figure 3). The feeding behavior of this arboreal animal has been argued as comparable to that of primates such as spider monkeys (Kays, 1999). This observation indicates that kinkajou could be ensuring seed dispersal in the canopy along RN2. Kinkajou might therefore contribute to forest resilience, through ecological and functional redundancy (Loiselle et al., 2007), by being an active disperser when other frugivores such as spider monkeys are lacking in the forest (Rother et al., 2016; Moreira et al., 2017). The maintenance of seed dispersal may also be complemented by large toucans, other important dispersers of Virola seeds (Howe and Vande Kerckhove, 1980, 1981; Holbrook and Loiselle, 2009; Ratiarison and Forget, 2013). In this study, however, using camera traps in the canopy, we failed to reveal high frequencies of diurnal species and canopy birds including toucans in fruiting Virola tree crowns. This may be due to the positions of the cameras in tree crowns as a study showed that wind and sun exposure can severely impact camera triggering, particularly in the canopy where weather conditions are more intense (Gregory et al., 2014). Camera positions were optimized to capture individuals eating fruits but camera exposure to environmental conditions was not considered. Further studies should thus pay more particular attention to camera positioning, and choose the best compromise between exposure and optimal position for observing diurnal animals (Bowler et al., 2017; Moore et al., 2021).
Manilkara Seed Dispersal System
The Manilkara 2014 masting event led to the accumulation of entire, uneaten fruit (55%) and non-dispersed seeds (63%) below parent trees standing in the forest along the RN2, with no apparent relation with the distance between trees and road, and a weak effect of the distance to the nearest urban area. Several factors could explain such high level of removal failure also called fruit waste (sensu Howe, 1980), including the abundance of fruit resources at the community level, the diversity and density of ground and arboreal consumers, as well as the saturating effect of the masting event among years and sites (Mendoza et al., 2015). However, when comparing fruit and seed fate at three contrasting forest habitats, it is apparent that fruit consumption and seed removal are more affected in the primate-dispersed species (Manilkara spp.) than in the more generalist species dispersed by both mammals and birds (Virola spp.) at RN2, the most stressed forest. Moreover, our results show that fruit consumption and seed removal rates decrease from the least disturbed (Nouragues) to the most disturbed sites (RN2 and Kaw). The low level of fruit consumption and seed removal at RN2 appeared to be comparable to Kaw. These results illustrate the indirect impacts of the RN2 in the area, which eases accessibility to the forest and promotes human activities such as hunting. The observed fruit waste in Manilkara at RN2 compared to Nouragues and Kaw is a common phenomenon within the Sapotaceae family level in stressed environmental conditions, especially defaunated ones (Gutierrez-Granados and Dirzo, 2010; Anzures-Dadda et al., 2011; Levi and Peres, 2013; Boissier et al., 2020). It suggests a lower population of specialized consumers of Manilkara in the canopy, e.g., howler monkeys and brown capuchins (Ratiarison and Forget, 2011), along with the satiation of the overall wildlife cotery on the ground beneath trees where whole fruit and seeds accumulate. Moreover, forest habitats along RN2 have important edge effects and the development of logging and ranching thanks to the construction of the RN2 favored the growth of secondary vegetation with pioneer and successional species bearing small-sized fruit. This concentrates the most favorable ecological conditions suitable for the establishment and persistence of small body-sized primates such as golden-handed tamarins Saguinus midas in disrupted forests (Pack et al., 1999), likely responsible for the greater proportion of removal failure. Indeed, because of their small size (Sivault et al., 2020), they can manipulate large fruits but will not ingest them and do not swallow large seeds. They rather spit them out after sucking the pulp, leading to their accumulation on the ground beneath tree crowns, which then attract ground-dwelling granivores such as large rodents and peccaries (Forget et al., 2007).
Frugivore Community
The apparent marginal effect of the National Road on fruit consumption and seed removal, particularly near the closest urban area of Saint-Georges, suggests that the wildlife community was not yet heavily affected by the road and the increased forest accessibility. An alternative hypothesis is that the lack of consumption and removal by the primary consumers and dispersers has been compensated by a greater abundance of secondary frugivores and granivores (Effiom et al., 2013; Moreira et al., 2017; Boissier et al., 2020). Indeed, most of the frugivorous species known to feed on Manilkara and Virola fruits and/or seeds were recorded in the study area. However, the main consumer of Virola fruits, the spider monkey, was never recorded. Frugivores such as large fruit-bats (e.g., Artibeus spp. or Carollia spp., Phyllostomatidae) known to forage and feed upon Manilkara fruit (Lobova et al., 2009) were not recorded either but this is likely due to the method, which was not designed to survey such flying species. Also, the quadrat method cannot evaluate the effect of frugivores that may swallow entire fruit (particularly Manilkara fruit), leaving no trace of consumption. However, such a bias is the same for all quadrats and depends on the presence of frugivores (nocturnal and terrestrial). During this study, the use of camera traps allowed enriching the diversity of known consumers by showing that deers and tapir consumed entire Manilkara fruits, possibly dispersing their seeds via regurgitation or in the feces (Feer et al., 2001), a feeding behavior that was missing in previous literature about their diet in the Guianas (Gayot et al., 2004; Hibert et al., 2011).
Primates are unlikely to forage across a fragmented forest landscape (Ratiarison and Forget, 2005) and the abundance of large terrestrial dispersers may decrease due to hunting (Moreira et al., 2017). Nonetheless, the human population of Saint-Georges remains limited (ca. 4,000 inhabitants), many of them inhabiting along the Oyapock river, where the hunting pressure is concentrated. The study was also carried out at a time when traffic was still relatively sparse since the cross-river international bridge had not yet opened. As a whole, road nuisances may remain relatively limited due to the small size of the human population.
The overall sharp differences in fruit handling, consumption, and seed fate beneath parent trees between Nouragues and RN2 reveal a change in the frugivorous community of the latter. The lower seed dispersal and accumulation of fruits below trees was likely due to the lack of key dispersers in the forest bordering the RN2. Hence, in line with the model of vulnerability, which predicts that population densities generally decline with increasing body mass, (Robinson and Redford, 1986), six species population densities decreased in the hunting area, i.e., three large primate species (spider monkey, howler monkey and capuchin) and the collared peccary (Tajacu pecari), black curassow (Crax alector) and gray-winged trumpeter (Psophia crepitans). Fruiting Virola and Manilkara trees are mostly visited and their fruits/seeds dispersed by arboreal mammals and birds, and the differences across study sites suggest that the primate community has been more impacted by environmental pressures along the RN2 compared to Nouragues and Kaw.
Limitations
The lack of effect on Manilkara fruit consumption between RN2 and Kaw suggests a similar disruption in the wildlife community, along with comparable anthropic pressures at both sites (hunting, logging, and fragmentation). This result is provisional given the small number of trees considered in the comparison (6 at Kaw vs. 30 trees at RN2), and a possible inter-annual effect, with a difference in Manilkara fruit production between 2010 and 2014. Still, results for Manilkara seed removal at RN2 are consistent with the general finding of low seed removal amongst Sapotaceae species at Kaw (Boissier et al., 2020).
To conduct a more thorough study of the fauna visiting trees, it would be necessary to implement camera traps in canopies at all different forests. The sampling must be improved with more fruiting trees by patch and forest type along the road, and at a greater distance (> 1 km) in the interior (see Miller et al., 2021). Cameras should be set up at different heights to gain greater information about arboreal animals, i.e., small to large, diurnal and nocturnal, visiting the tree crowns or not (Moore et al., 2021; Zhu et al., 2021). We expect that small frugivorous vertebrates are also important, contributing to the resilience of animal-plant interaction networks in stressed and endangered forest habitats (Carreira et al., 2020). Such a study should also be repeated during other years at the forest patches, and across forests, to minimize month effect and year effects that most likely would alter results and comparisons across sites, especially during mast fruiting events of Manilkara species (Norden et al., 2007; Mendoza et al., 2015). Furthermore, since the bridge opened to traffic in 2017, it would be interesting to study how resilient the wildlife has been, and to compare how frugivorous species and trees responded to the expected greater road traffic, which has barely been investigated during impact assessments (Vilela et al., 2020).
Conclusion
Based on the current data, the study does not indicate a significant effect of distance to the urban area and to the road on frugivorous activity and seed dispersal. This is likely due to a compensatory effect of small mammals replacing large and absent ones in the most impacted forests. However, the comparison across forests revealed significant differences in the type of fruit handling, and the rate of fruit consumption and dispersal rate among tree species, despite only a small number of sampled trees being included for one study tree species (Manilkara). Tropical biodiversity conservation research should take into consideration the threats that new bisecting roads (such as RN2 in the last Western forest block) represent for a region that is an excellent natural laboratory to understand the process of human-induced biodiversity degradation and the associated species responses (Kocher et al., 2017, 2022). A decade after the road was asphalted and open to traffic, this study indicates a moderate effect of the road on frugivorous activity and ecological services. This first round of observations would need to be more thorough to explain the origins and causes of the differences observed from an environmental perspective, as well as to evaluate how flora and fauna differ across forest stands within the study sites. Repeated measurements of ecological services throughout time, at 5–10 year intervals, at the same forest patches, and possibly at the same individual trees and species, across forest sites, would allow evaluation of the effects of inter-annual variability and masting years (Norden et al., 2007; Mendoza et al., 2015, 2018) on fruit consumption and seed removal, and the expected greater fruit waste (sensu Howe, 1980) in primate-impoverished animal communities.
The tropical rainforest is a complex ecosystem that relies on plant-animal interactions occurring at each step of the life cycle of a plant (Terborgh et al., 2008). It is therefore vital to urgently evaluate the effects of anthropogenic pressure on animal communities, due to the change of interactions they can induce and to the cascading effects it will have at different scales—local, regional and global. The frugivorous vertebrates, by their role as seed dispersers, are a crucial factor of change and efficiency of seed recruitment, which affect plant diversity in the short term. Indeed, the lower seedling recruitment is below the reproductive tree, the higher species coexistence will be (Terborgh et al., 2008). The impoverishment of the community of large frugivores, because of various anthropogenic pressures (hunting, fragmentation, logging), leads to a downsizing effect at the community level, with an increased population of small-sized frugivores. Within several years or decades, defaunation combined with downsizing effects will have consequences on specialized large-seeded tree species such as Virola and Manilkara that will fail to recruit (Levi and Peres, 2013), being progressively replaced by generalized small-seeded and small animal-dispersed tree species (Moreira et al., 2017). Conservation programs have to consider interactions between plants and animals to maintain forest diversity despite human pressures. The decrease in hunting pressure on dispersers and increasing connectivity between populations are key points for the conservation of the diversity of plant-animal interactions.
Data Availability Statement
The raw data supporting the conclusions of this article are available as Supplementary Tables 5–8. Pictures from camera traps are included in https://www.agouti.eu and are available upon requests directed to the corresponding author(s).
Ethics Statement
Ethical review and approval was not required for the animal study because animals were not captured or manipulated.
Author Contributions
OC, OB, AB, and P-MF conceived and designed the fruit surveys at all sites. OC, EG, and P-MF conceived the camera trap protocol at RN2. OC, AA-D, OB, CMD, FF, IM, EG, and P-MF performed the fruit and seed surveys. OC, MD, and P-MF performed data analysis and interpretation. OC, MD, OB, IM, and P-MF wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was carried out within the framework of the Oyapock Human-Nature Observatory of the Centre National de la Recherche Scientifique. It was supported by the OHM Oyapock via the Labex DRIIHM/IRDHEI and ANR-11-LABX-0010, and the UMR MECADEV 7179 CNRS-MNHN. OC was supported with a Master grant from the Fondation Pour la Recherche sur la Biodiversité. IM was supported by the Spanish Ministry of Education (reference EX2009-0711) during fieldwork and by the Grant PID2020-115129RJI00 by MCIN/AEI/10.13039/501100011033 during the writing of the manuscript. OB was supported by UMR 7179 CNRS-MNHN and Ecole Normale Supérieure. CMD was supported by a Ph.D. scholarship from the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES)” and “Ciência sem Fronteiras” Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Anaig Le Guen and Damien Davy of the UMS Laboratoire Ecologie, Evolution, Interactions des Systèmes Amazoniens (LEEISA) for offering help with logistics and accommodation in Saint-George. We thank Cécile Richard-Hansen (Office Français de la Biodiversité, formerly ONCFS) who provided security enclosures for the Reconyx ® camera trap, and for her advice and discussion during protocol setting. We are also especially thankful to Patrick Ho in Bazar de l’Oyapock, in Saint-Georges, for establishing a kind and trustable relationship with the hunter population, who did not destroy the survey material when detecting it during the course of their walks at the study forest patches. Nadia Patel reviewed the English of the text.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.805376/full#supplementary-material
References
Alamgir, M., Campbell, M. J., Sloan, S., Engert, J., Word, J., and Laurance, W. F. (2020). Emerging challenges for sustainable development and forest conservation in Sarawak, Borneo. PLoS One 15:e0229614. doi: 10.1371/journal.pone.0229614
Alamgir, M., Campbell, M. J., Sloan, S., Goosem, M., Clements, G. R., Mahmoud, M. I., et al. (2017). Economic, socio-political and environmental risks of road development in the tropics. Curr. Biol. 27, R1130–R1140. doi: 10.1016/j.cub.2017.08.067
Anzures-Dadda, A., Andresen, E., Martinez, M. L., and Manson, R. H. (2011). Absence of Howlers (Alouatta palliata) influences tree seedling densities in tropical rain forest fragments in Southern Mexico. Int. J. Primatol. 32, 634–651. doi: 10.1007/s10764-011-9492-0
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Benítez-López, A., Santini, L., Schipper, A. M., Busana, M., and Huijbregts, M. A. J. (2019). Intact but empty forests? Patterns of hunting-induced mammal defaunation in the tropics. PLoS Biol. 17:e3000247. doi: 10.1371/journal.pbio.3000247
Boissier, O., Bouiges, A., Mendoza, I., Feer, F., and Forget, P.-M. (2014). Rapid assessment of seed removal and frugivore activity as a tool for monitoring the health status of tropical forests. Biotropica 46, 633–641. doi: 10.1111/btp.12134
Boissier, O., Feer, F., Henry, P.-Y., and Forget, P.-M. (2020). Modifications of the rain forest frugivore community are associated with reduced seed removal at the community level. Ecol. Appl. 30:e02086. doi: 10.1002/eap.2086
Boudoux d’Hautefeuille, M. (2010). La frontière et ses échelles: les enjeux d’un pont transfrontalier entre la Guyane française et le Brésil/Borders and scales: the challenges of a cross-border bridge between French Guiana and Brazil. Cybergeo Eur. J. Geogr.
Bowler, M. T., Tobler, M. W., Endress, B. A., Gilmore, M. P., and Anderson, M. J. (2017). Estimating mammalian species richness and occupancy in tropical forest canopies with arboreal camera traps. Remote Sens. Ecol. Conserv. 3, 146–157. doi: 10.1002/rse2.35
Brodie, J. F., and Fragoso, J. M. V. (2020). Understanding the distribution of bushmeat hunting effort across landscapes by testing hypotheses about human foraging. Conserv. Biol. 35, 1009–1018. doi: 10.1111/cobi.13612
Carreira, D. C., Dáttilo, W., Bruno, D. L., Percequillo, A. R., Ferraz, K. M. P. M. B., and Galetti, M. (2020). Small vertebrates are key elements in the frugivory networks of a hyperdiverse tropical forest. Sci. Rep. 10:10594. doi: 10.1038/s41598-020-67326-6
Chao, A., Gotelli, N. J., Hsieh, T. C., Sander, E. L., Ma, K. H., Colwell, R. K., et al. (2014). Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67. doi: 10.1890/13-0133.1
Chauvet, S., Feer, F., and Forget, P. M. (2004). Seed fate of two Sapotaceae species in a Guianan rain forest in the context of escape and satiation hypotheses. J. Trop. Ecol. 20, 1–9. doi: 10.1017/s0266467404006121
Clements, G. R., Aziz, S. A., Bulan, R., Giam, X., Bentrupperbaumer, J., Goosem, M., et al. (2018). Not everyone wants roads: assessing indigenous people’s support for roads in a globally important tiger conservation landscape. Hum. Ecol. 46, 909–915. doi: 10.1007/s10745-018-0029-4
Clements, G. R., Lynam, A. J., Gaveau, D., Yap, W. L., Lhota, S., Goosem, M., et al. (2014). Where and how are roads endangering mammals in Southeast Asia’s forests? PLoS One 9:e115376. doi: 10.1371/journal.pone.0115376
Coffin, A. W. (2007). From roadkill to road ecology: a review of the ecological effects of roads. J. Transp. Geogr. 15, 396–406. doi: 10.1016/j.jtrangeo.2006.11.006
Corlett, R. T. (2017). Frugivory and seed dispersal by vertebrates in tropical and subtropical Asia: an update. Glob. Ecol. Conserv. 11, 1–22. doi: 10.1016/j.gecco.2017.04.007
de Thoisy, B., Fayad, I., Clément, L., Barrioz, S., Poirier, E., and Gond, V. (2016). Predators, prey and habitat structure: can key conservation areas and early signs of population collapse be detected in neotropical forests? PLoS One 11:e0165362. doi: 10.1371/journal.pone.0165362
de Thoisy, B., Renoux, F., and Julliot, C. (2005). Hunting in northern French Guiana and its impacts on primate communities. Oryx 39, 149–157. doi: 10.1017/S0030605305000384
de Thoisy, B., Richard-Hansen, C., Goguillon, B., Joubert, P., Obstancias, J., Winterton, P., et al. (2010). Rapid evaluation of threats to biodiversity: human footprint score and large vertebrate species responses in French Guiana. Biodivers. Conserv. 19, 1567–1584. doi: 10.1007/s10531-010-9787-z
Dugger, P. J., Blendinger, P. G., Böhning-Gaese, K., Chama, L., Correia, M., Dehling, D. M., et al. (2019). Seed-dispersal networks are more specialized in the Neotropics than in the Afrotropics. Glob. Ecol. Biogeogr. 28, 248–261. doi: 10.1111/geb.12833
Effiom, E. O., Nuñez-Iturri, G., Smith, H. G., Ottosson, U., and Olsson, O. (2013). Bushmeat hunting changes regeneration of African rainforests. Proc. R. Soc. B Biol. Sci. 280:20130246. doi: 10.1098/rspb.2013.0246
Emer, C., Jordano, P., Pizo, M. A., Ribeiro, M. C., da Silva, F. R., and Galetti, M. (2020). Seed dispersal networks in tropical forest fragments: area effects, remnant species, and interaction diversity. Biotropica 52, 81–89. doi: 10.1111/btp.12738
Fahrig, L., and Rytwinski, T. (2009). Effects of roads on animal abundance: an empirical review and synthesis. Ecol. Soc. 14:21. doi: 10.5751/ES-02815-140121
Feer, F., Henry, O., Forget, P.-M., and Gayot, M. (2001). “Frugivory and seed dispersal by terrestrial mammals,” in Nouragues: Dynamics and Plant-Animal Interactions in a Neotropical Rainforest, eds F. Bongers, P. Charles-Dominique, P.-M. Forget, and M. Théry (Dordrecht: Springer Netherlands), 227–232.
Forget, P.-M., Dennis, A. J., Mazer, S. J., Jansen, P. A., Kitamura, S., Lambert, J. E., et al. (2007). “Seed allometry and disperser assemblages in tropical rainforests: a comparison of four floras on different continents,” in Seed Dispersal: Theory and its Application in a Changing World, eds A. Dennis, E. W. Schupp, R. Green, and D. Wescott (Wallingford: CABI Publishing), 5–36.
Forget, P. M., and Sabatier, D. (1997). Dynamics of the seedling shadow of a frugivore-dispersed tree species in French Guiana. J. Trop. Ecol. 13, 767–773. doi: 10.1017/S0266467400010920
Forman, R. T. T., and Alexander, L. E. (1998). Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 29, 207–231. doi: 10.1146/annurev.ecolsys.29.1.207
Gallego-Zamorano, J., Benítez-López, A., Santini, L., Hilbers, J. P., Huijbregts, M. A. J., and Schipper, A. M. (2020). Combined effects of land use and hunting on distributions of tropical mammals. Conserv. Biol. 34, 1271–1280. doi: 10.1111/cobi.13459
Gayot, M., Henry, O., Dubost, G., and Sabatier, D. (2004). Comparative diet of the two forest cervids of the genus Mazama in French Guiana. J. Trop. Ecol. 20, 31–43. doi: 10.1017/S0266467404006157
Gregory, T., Carrasco Rueda, F., Deichmann, J., Kolowski, J., and Alonso, A. (2014). Arboreal camera trapping: taking a proven method to new heights. Methods Ecol. Evol. 5, 443–451. doi: 10.1111/2041-210x.12177
Gregory, T., Carrasco-Rueda, F., Deichmann, J., Kolowski, J., and Alonso, A. (2017). Primate response to natural gas pipeline construction in the Peruvian Amazon. Biotropica 49, 249–255. doi: 10.1111/btp.12406
Grenand, F. (2011). Un pont entre la France et le Brésil: l’Observatoire hommes/milieux sur le fleuve oyapock. Rayonnement CNRS 56, 41–47.
Guitet, S., Pélissier, R., Brunaux, O., Jaouen, G., and Sabatier, D. (2015). Geomorphological landscape features explain floristic patterns in French Guiana rainforest. Biodivers. Conserv. 24, 1215–1237. doi: 10.1007/s10531-014-0854-8
Guitet, S., Sabatier, D., Brunaux, O., Couteron, P., Denis, T., Freycon, V., et al. (2018). Disturbance regimes drive the diversity of regional floristic pools across Guianan rainforest landscapes. Sci. Rep. 8:3872. doi: 10.1038/s41598-018-22209-9
Gutierrez-Granados, G., and Dirzo, R. (2010). Indirect effects of timber extraction on plant recruitment and diversity via reductions in abundance of frugivorous spider monkeys. J. Trop. Ecol. 26, 45–52. doi: 10.1017/s0266467409990307
Hambuckers, A., Trolliet, F., Simon, A., Cazetta, E., and Rocha-Santos, L. (2020). Seed removal rates in forest remnants respond to forest loss at the landscape scale. Forests 11:1144. doi: 10.3390/f11111144
Hambuckers, J., Dauvrin, A., Trolliet, F., Evrard, Q., Forget, P. M., and Hambuckers, A. (2017). How can seed removal rates of zoochoric tree species be assessed quickly and accurately? For. Ecol. Manag. 403, 152–160. doi: 10.1016/j.foreco.2017.07.042
Hammond, D. S. (2005). “Socio-economic aspects of Guian Shield forest use,” in Tropical Forests of the Guiana Shield: Ancient Forests in a Modern World, ed. D. S. Hammond (Wallington: CABI International), 381–480.
Hibert, F., Sabatier, D., Andrivot, J., Scotti-Saintagne, C., Gonzalez, S., Prévost, M.-F., et al. (2011). Botany, genetics and ethnobotany: a crossed investigation on the elusive tapir’s diet in French Guiana. PLoS One 6:e25850. doi: 10.1371/journal.pone.0025850
Holbrook, K. M., and Loiselle, B. A. (2009). Dispersal in a neotropical tree, Virola flexuosa (Myristicaceae): does hunting of large vertebrates limit seed removal? Ecology 90, 1449–1455. doi: 10.1890/08-1332.1
Howe, H. F. (1980). Monkey dispersal and waste of a neotropical fruit. Ecology 61, 944–959. doi: 10.2307/1936763
Howe, H. F. (1993). Specialized and generalized dispersal systems: where does ‘the paradigm’ stand? Vegetatio 107-108, 3–13. doi: 10.1007/978-94-011-1749-4_1
Howe, H. F., and Vande Kerckhove, G. A. (1980). Nutmeg dispersal by tropical birds. Science 210, 925–927. doi: 10.1126/science.210.4472.925
Howe, H. F., and Vande Kerckhove, G. A. (1981). Removal of wild nutmeg (Virola surinamensis) crops by birds. Ecology 62, 1093–1106. doi: 10.2307/1937007
Hsieh, T. C., Ma, K. H., and Chao, A. (2016). iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 7, 1451–1456. doi: 10.1111/2041-210X.12613
Huber, O., Foster, M. N., and Pires, T. C. A. (2002). Conservation Priorities for the Guayana Shield: 2002 Consensus. Washington, DC: Conservation International, Center for Applied Biodiversity Science.
Kays, R. W. (1999). Food preferences of Kinkajou (Potos flavus): a frugivorous carnivore. J. Mammal. 80, 589–599. doi: 10.2307/1383303
Kitamura, S., and Poonswad, P. (2013). Nutmeg-vertebrate interactions in the Asia-Pacific region: importance of frugivores for seed dispersal in Myristicaceae. Trop. Conserv. Sci. 6, 608–636. doi: 10.1177/194008291300600503
Kocher, A., Cornuault, J., Gantier, J.-C., Manzi, S., Chavy, A., Girod, R., et al. (2022). Biodiversity and vector-borne diseases: host dilution and vector amplification occur simultaneously for Amazonian leishmaniases. Mol. Ecol. doi: 10.1111/mec.16341 [Online ahead of print].
Kocher, A., de Thoisy, B., Catzeflis, F., Valière, S., Bañuls, A.-L., and Murienne, J. (2017). iDNA screening: disease vectors as vertebrate samplers. Mol. Ecol. 26, 6478–6486. doi: 10.1111/mec.14362
Laurance, W. F., and Arrea, I. B. (2017). Roads to riches or ruin? Science 358, 442–444. doi: 10.1126/science.aao0312
Laurance, W. F., Clements, G. R., Sloan, S., O’Connell, C. S., Mueller, N. D., Goosem, M., et al. (2014). A global strategy for road building. Nature 513, 229–232. doi: 10.1038/nature13717
Laurance, W. F., Cochrane, M. A., Bergen, S., Fearnside, P. M., Delamonica, P., Barber, C., et al. (2001). The future of the Brazilian Amazon. Science 291, 438–439. doi: 10.1126/science.291.5503.438
Laurance, W. F., Goosem, M., and Laurance, S. G. (2009). Impacts of roads and linear clearings on tropical forests. Trends Ecol. Evol. 24, 659–669. doi: 10.1016/j.tree.2009.06.009
Laurance, W. F., Useche, D. C., Rendeiro, J., Kalka, M., Bradshaw, C. J. A., Sloan, S. P., et al. (2012). Averting biodiversity collapse in tropical forest protected areas. Nature 489, 290–294. doi: 10.1038/nature11318
Levi, T., and Peres, C. A. (2013). Dispersal vacuum in the seedling recruitment of a primate-dispersed Amazonian tree. Biol. Conserv. 163, 99–106. doi: 10.1016/j.biocon.2013.03.016
Lobova, T. A., Geiselman, C. K., Mori, S. A., and Garden, N. Y. B. (2009). Seed Dispersal by Bats in the Neotropics. New York, NY: New York Botanical Garden.
Loiselle, B., Blendinger, P., Blake, J., and Ryder, T. (2007). “Ecological redundancy in seed dispersal systems: a comparison between manakins (Aves: Pipridae) in two tropical forests,” in Seed Dispersal: Theory and its Application in a Changing World, eds A. J. Dennis, E. W. Schupp, R. J. Green, and D. A. Westcott (Wallingford: CAB International), 178–195.
Markl, J. S., Schleuning, M., Forget, P. M., Jordano, P., Lambert, J. E., Traveset, A., et al. (2012). Meta-analysis of the effects of human disturbance on seed dispersal by animals. Conserv. Biol. 26, 1072–1081. doi: 10.1111/j.1523-1739.2012.01927.x
Mendoza, I., Condit, R. S., Wright, S. J., Caubère, A., Châtelet, P., Hardy, I., et al. (2018). Inter-annual variability of fruit timing and quantity at Nouragues (French Guiana): insights from hierarchical Bayesian analyses. Biotropica 50, 431–441. doi: 10.1111/btp.12560
Mendoza, I., Martin, G., Caubere, A., Chatelet, P., Hardy, I., Jouard, S., et al. (2015). Does masting result in frugivore satiation? A test with Manilkara trees in French Guiana. J. Trop. Ecol. 31, 553–556. doi: 10.1017/S0266467415000425
Miller, S. C., Wiethase, J. H., Motove Etingue, A., Franklin, E., Fero, M., Wolfe, J. D., et al. (2021). Interactive effects of elevation and newly paved road on avian community composition in a scientific reserve, Bioko Island, Equatorial Guinea. Biotropica 53, 1646–1663. doi: 10.1111/btp.13014
Moore, J. F., Soanes, K., Balbuena, D., Beirne, C., Bowler, M., Carrasco-Rueda, F., et al. (2021). The potential and practice of arboreal camera trapping. Methods Ecol. Evol. 12, 1768–1779. doi: 10.1111/2041-210X.13666
Moreira, J. I., Riba-Hernández, P., and Lobo, J. A. (2017). Toucans (Ramphastos ambiguus) facilitate resilience against seed dispersal limitation to a large-seeded tree (Virola surinamensis) in a human-modified landscape. Biotropica 49, 502–510. doi: 10.1111/btp.12427
Naniwadekar, R., Chaplod, S., Datta, A., Rathore, A., and Sridhar, H. (2019). Large frugivores matter: insights from network and seed dispersal effectiveness approaches. J. Anim. Ecol. 88, 1250–1262. doi: 10.1111/1365-2656.13005
Ng, S. J., Dole, J. W., Sauvajot, R. M., Riley, S. P. D., and Valone, T. J. (2004). Use of highway undercrossings by wildlife in southern California. Biol. Conserv. 115, 499–507. doi: 10.1016/S0006-3207(03)00166-6
Nicolle, S., and Boudoux d’Hautefeuille, M. (2014). Anticiper la Route: Étude de cas dans l’est de la Guyane Française. Available Online at: https://journals.openedition.org/vertigo/14677 (accessed August 25, 2020).
Norden, N., Chave, J., Belbenoit, P., Caubere, A., Chatelet, P., Forget, P.-M., et al. (2007). Mast fruiting is a frequent strategy in woody species of Eastern South America. PLoS One 2:e1079. doi: 10.1371/journal.pone.0001079
Nuñez-Iturri, G., and Howe, H. F. (2007). Bushmeat and the fate of trees with seeds dispersed by large primates in a lowland rain forest in western Amazonia. Biotropica 39, 348–354. doi: 10.1111/j.1744-7429.2007.00276.x
O’Bryan, C. J., Garnett, S. T., Fa, J. E., Leiper, I., Rehbein, J., and Fernández-Llamazares, Á, et al. (2020). The importance of indigenous peoples’ lands for the conservation of terrestrial mammals. Conserv. Biol. 35, 1002–1008. doi: 10.1111/cobi.13620
Pack, K. S., Henry, O., and Sabatier, D. (1999). The insectivorous-frugivorous diet of the golden-handed tamarin (Saguinus midas midas) in French Guiana. Folia Primatol. 70, 1–7.
Pardo, L. E., Campbell, M. J., Cove, M. V., Edwards, W., Clements, G. R., and Laurance, W. F. (2019). Land management strategies can increase oil palm plantation use by some terrestrial mammals in Colombia. Sci. Rep. 9:7812. doi: 10.1038/s41598-019-44288-y
Peres, C. A. (2001). Synergistic effects of subsistence hunting and habitat fragmentation on Amazonian forest vertebrates. Conserv. Biol. 15, 1490–1505.
Pérez, P., and Archambeau, O. (2012). Architectures et Paysages de Saint-Georges de l’Oyapock. OHM Oyapock, CNRS Guyane (Cayenne). French Guiana: Saint-Georges de l’Oyapock.
Picart, L., Forget, P.-M., D’Haese, C. A., Daugeron, C., Beni, S., Bounzel, R., et al. (2014). The CAFOTROP method: an improved rope-climbing method for access and movement in the canopy to study biodiversity. Ecotropica 20, 45–52.
R Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ratiarison, S., and Forget, P. M. (2005). Frugivores and seed removal at Tetragastris altissima (Burseraceae) in a fragmented forested landscape of French Guiana. J. Trop. Ecol. 21, 501–508. doi: 10.1017/s0266467405002518
Ratiarison, S., and Forget, P.-M. (2011). Fruit availability, frugivore satiation and seed removal in 2 primate-dispersed tree species. Integr. Zool. 6, 178–194. doi: 10.1111/j.1749-4877.2011.00243.x
Ratiarison, S., and Forget, P.-M. (2013). The role of frugivores in determining seed removal and dispersal in the Neotropical nutmeg. Trop. Conserv. Sci. 6, 690–704. doi: 10.1177/194008291300600508
Robinson, J. G., and Redford, K. H. (1986). Body size, diet, and population density of neotropical forest mammals. Am. Nat. 128, 665–680. doi: 10.1086/284596
Rodrigues, A. S. L., Andelman, S. J., Bakarr, M. I., Boitani, L., Brooks, T. M., Cowling, R. M., et al. (2003). Global Gap Analysis: Towards a Representative Network of Protected Areas. Advances in Applied Biodiversity Science, Vol. 5. Washington DC: Conservation International.
Rother, D. C., Pizo, M. A., and Jordano, P. (2016). Variation in seed dispersal effectiveness: the redundancy of consequences in diversified tropical frugivore assemblages. Oikos 125, 336–342. doi: 10.1111/oik.02629
Sabatier, D. (1997). Description et biologie d’une nouvelle espèce de Virola (Myristicaceae) de Guyane. Adansonia Ser. 3, 273–278.
Sales, L., Culot, L., and Pires, M. M. (2020). Climate niche mismatch and the collapse of primate seed dispersal services in the Amazon. Biol. Conserv. 247:108628. doi: 10.1016/j.biocon.2020.108628
Shi, H., Shi, T., Yang, Z., Wang, Z., Han, F., and Wang, C. (2018). Effect of roads on ecological corridors used for wildlife movement in a natural heritage site. Sustainability 10:2725. doi: 10.3390/su10082725
Sivault, E., Herrel, A., Forget, P.-M., Fabre, A.-C., and Bretagnolle, F. (2020). “The body weight and skull measurements predict seed dispersal capacity in bat, primate and carnivore species,” in Proceedings of the Seed Dispersal in the Atnthropocene - 7th Frugivores and Seed Disersal Symposium, eds S. Prasad, K. R. McConkey, and E. Cazetta (Corbett).
Sollmann, R., Gardner, B., Williams, K. A., Gilbert, A. T., and Veit, R. R. (2016). A hierarchical distance sampling model to estimate abundance and covariate associations of species and communities. Methods Ecol. Evol. 7, 529–537. doi: 10.1111/2041-210X.12518
Stevenson, P. R. (2016). Neotropical primate communities: effects of disturbance, resource production and forest type heterogeneity. Am. J. Primatol. 78, 391–401. doi: 10.1002/ajp.22518
Stevenson, P. R., Link, A., and Ramírez, B. H. (2005). Frugivory and seed fate in Bursera inversa (Burseraceae) at Tinigua Park, Colombia: implications for primate conservation. Biotropica 37, 431–438. doi: 10.1111/j.1744-7429.2005.00057.x
Terborgh, J., Nuñez-Iturri, G., Pitman, N. C. A., Valverde, F. H. C., Alvarez, P., Swamy, V., et al. (2008). Tree recruitment in an empty forest. Ecology 89, 1757–1768. doi: 10.1890/07-0479.1
Trombulak, S. C., and Frissell, C. A. (2000). Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 14, 18–30. doi: 10.1046/j.1523-1739.2000.99084.x
Vilela, T., Malky Harb, A., Bruner, A., Laísa da Silva Arruda, V., Ribeiro, V., Auxiliadora Costa Alencar, A., et al. (2020). A better Amazon road network for people and the environment. Proc. Natl. Acad. Sci. U.S.A. 117, 7095–7102. doi: 10.1073/pnas.1910853117
Wright, S. J., and Duber, H. C. (2001). Poachers and forest fragmentation alter seed dispersal, seed survival, and seedling recruitment in the palm Attalea butyraceae, with implications for tropical tree diversity. Biotropica 33, 583–595. doi: 10.1111/j.1744-7429.2001.tb00217.x
Zhang, S.-Y. (1995). Activity and ranging patterns in relation to fruit utilization by brown capuchins (Cebus apella) in French Guiana. Int. J. Primatol. 16, 489–507. doi: 10.1007/BF02735799
Keywords: seed dispersal, anthropogenic disturbances, Road “Nationale 2”, Manilkara bidentata, Manilkara huberi, Virola kwatae, Virola michelii
Citation: Coutant O, Boissier O, Ducrettet M, Albert-Daviaud A, Bouiges A, Dracxler CM, Feer F, Mendoza I, Guilbert E and Forget P-M (2022) Roads Disrupt Frugivory and Seed Removal in Tropical Animal-Dispersed Plants in French Guiana. Front. Ecol. Evol. 10:805376. doi: 10.3389/fevo.2022.805376
Received: 30 October 2021; Accepted: 08 March 2022;
Published: 26 April 2022.
Edited by:
Omer Nevo, German Centre for Integrative Biodiversity Research (iDiv), GermanyReviewed by:
Ben T. Hirsch, James Cook University, AustraliaKim McConkey, National Institute of Advanced Studies, India
Copyright © 2022 Coutant, Boissier, Ducrettet, Albert-Daviaud, Bouiges, Dracxler, Feer, Mendoza, Guilbert and Forget. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pierre-Michel Forget, pierre-michel.forget@mnhn.fr
 Opale Coutant
Opale Coutant Olivier Boissier
Olivier Boissier Manon Ducrettet1
Manon Ducrettet1  Caroline Marques Dracxler
Caroline Marques Dracxler Irene Mendoza
Irene Mendoza Eric Guilbert
Eric Guilbert Pierre-Michel Forget
Pierre-Michel Forget